Learn about Lithium-ion Battery for Electric Vehicle – Part-1
Let us start with the basics of Lithium-ion Battery for electric vehicles. Next, we will deal with the metal Lithium, Raw Materials for Li-ion battery, Structure of the fundamental Li-ion battery cell and The Working principle of the li-ion battery.
1.0: Some notes about metal lithium
To start with let’s have some ideas about the metal Lithium. Lithium is an element discovered by Johan August Arfvedson in 1817. The name is derived from the Greek “lithos” meaning stone. The chemical symbol is “Li” and atomic number is 3. It is soft and silvery white alkali metal. Lithium is highly reactive and flammable. Lithium is the 35th most abundant element on earth. Earth has a stock of approximately 230 billion tons of lithium. Lithium is available in ocean water and commonly extracted from brines. The largest Lithium sources in the world are Chile and Australia and US, Argentina and China are also some of the major producers.

Fig_1: Lithium from Brines
Fig_1A below shows Lithium-ion world production as predicted up to the year 2020.
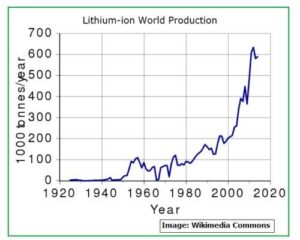
Fig_1A: Lithium-Ion world production
Commercial Lithium is recovered by evaporating brines collected from salars and salt lakes in evaporation ponds. It takes a year or more to recover Lithium this way and leaves behind lots of salt waste. There are the latest technologies and processes that offer more profitable options for Lithium extraction.
Lithium is sometimes precipitated as Lithium Phosphate for faster precipitation and then Lithium Phosphate is converted into battery-grade Lithium Hydroxide through an electrochemical process.
1.1: Are Lithium-Ion battery used in electric vehicles different from conventional gas-powered vehicles?
Electric-vehicle batteries are different from gasoline-based automobiles which use SLI Batteries (Starting, Lighting, and Ignition). The Electric Vehicle batteries are designed to give power over prolonged periods of time so that the vehicle can run a long distance without recharge. Deep-cycle high ampere-hour capacity batteries are used instead of SLI batteries for EV applications.
The battery in an electric vehicle must deliver power to drive the electric motor. Electric Vehicle batteries are a secondary (rechargeable) battery.
Lithium-ion batteries are used in Electric Vehicles due to their high energy density, high power density, long lifespan and environmental friendliness.
These batteries are excellent for energy storage solutions.
We will now peep inside the Lithium-ion battery specifically manufactured for electric vehicles to find out their typical raw materials requirements and internal structures
1.2: Lithium-ion Battery Raw Materials:
Fig_2 below has details of all the metals which are used to make lithium-ion battery. Some of them are used to make the Anode and most are used to manufacture Cathode. Apart from Lithium, the most used metals are Nickel, Cobalt and Aluminum.

Fig_2: Raw Materials used to make Li-ion Battery
1.3: Structure of a typical Lithium-ion Battery Cell:
Fig_3 below shows the internal structure of a typical Lithium-ion battery cell.
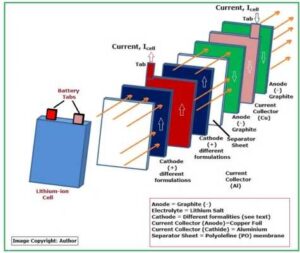
Fig_3: Structure of a Lithium Cell
Each lithium-ion cell contains THREE major parts:
- Anode (Graphite) negative electrode
- Electrolyte (Lithium Salt)
- Cathode (Differently Formulated – positive electrode)
Apart from these 3 items, there are Current Collectors, Separator sheets and cover for the cell. Here are the details of all these parts:
1.4: About Current Collectors:
Typically, Copper Foil is used as the negative electrode, or the anode current collectors and aluminum is used as the positive electrode or the cathode current collectors.Aluminum oxidizes more easily than copper to form metal oxide for electrochemical oxidation. Aluminum is also prone to galvanic corrosion in contact with copper.
In fact, aluminum oxide film forms on the aluminum base metal. Aluminum oxide is known to be chemically and electrochemically a stable film. Therefore, the oxide film of the aluminum can be considered as the auxiliary (cathode) electrode in Li-ion battery, in which the exchange current is very high compared to the copper at the anode side. The net exchange current of the aluminum oxide is equal to zero. In other words, the aluminum oxide film doesn’t polarize. This is the reason which makes aluminum oxide film an excellent current collector.
A rolled foil (RA-type), made from wrought Cu is generally used for Li-ion battery used for electric vehicles as the requirement is high-energy and high-power. Aluminum foils are used as the cathode current collector of secondary Li-ion batteries. Currently, the anode is comprised of a Graphite mixture, while the cathode combines Lithium and other choice metals, and all materials in a battery have a theoretical energy density. With Lithium-ion, the anode is well optimized, and design changes will yield little to no significant improvements in performance. On the other hand, the cathode material is wide open to enhancements and explains why today’s battery research is so heavily focused on this area.
Cathode Active Materials:
Cathode Active Materials are the main elements having different compositions which are the basic requirements for manufacturing positive electrodes for battery cells. The cathode materials are comprised of cobalt, nickel and manganese in the crystal structure forming a multi-metal oxide material to which lithium is added. The batteries manufactured specifically for electric vehicles use a variety of Cathode materials to cater to different application requirements for high energy density and high load capacity.
5 types of Cathode materials are used to manufacture Lithium-ion batteries for electric vehicles:
- NMC (NCM) – Lithium Nickel Cobalt Manganese Oxide (LiNiCoMnO2) – 29%
- LCO – Lithium Cobalt Oxide (LiCoO2) – 26%
- LFP – Lithium Iron Phosphate (LiFePO4) – 23%
- LMO – Lithium Manganese Oxide (LiMn2O4) – 12%
- NCA – Lithium Nickel Cobalt Aluminum Oxide (LiNiCoAlO2)- 10%
Apart from Lithium, some other metals are also used to manufacture Cathode. In fact, there are many varieties of Cathodes with different formulations.
Fig_3A Tabulates the formulations used in some of the major ones.
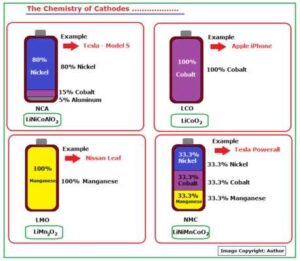
Fig_3A: The Chemistry of Cathodes
About Separator Materials:
The safety of a li-ion battery cell is dependent on the separator. This is a thin, porous membrane which separates the cell cathode and anode. The separator prevents physical contact between cathode and anode. The challenge is to manufacture a separator which will be mechanically strong and at the same time porous to facilitate transport properties. Separators are normally manufactured out of simple plastic films that have the right pore size to allow ions to flow through and block the other components. Polypropylene (PP), Polyethylene (PE), and ceramic-embedded battery separators is found to have the required porosity, lightness and durability.
1.5: Li-ion Battery working principle:
We have explained above that the primary components of Li-ion batteries are Anode, Cathode and Separator.
Li-ions intercalate and de-intercalate between the anode and cathode during charging and discharging process. Intercalation in Li-on batteries will take place only during the charging and discharging process and not when the battery is idle or when the battery is dead. A Li-on battery, like all batteries, consists of a positive electrode, negative electrode, and electrolytes. During discharging, the positive Lithium ion moves from the negative electrode (usually graphite) and enters the positive electrode (usually lithium oxide) through the electrolyte solution (made of organic solvent in solid or liquid form). During charging, the opposite of this process occurs, which is the reason why this is known as a reversible process.
This is clearly shown in Fig_4 below:
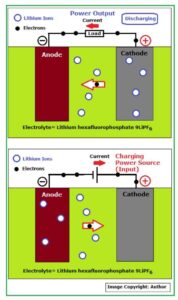
Fig_4: Schematic of Li-ion battery working principle
During charge / discharge procedure, a chemical reaction caused by or accompanied by an electrical current (electron flow) takes place as shown in the above figure.
The basic functions of the three components in a Li-ion battery are as below:
- The Anode or negative electrode: This is the reducing or fuel electrode which releases electrons to the external circuit and oxidizes during the electrochemical reaction.
- The Cathode or positive electrode: This is the oxidizing electrode which accepts electrons from the external circuit and reduces during the electrochemical reaction.
- The electrolyte is the ionic conductor: This provides the medium for transfer of charge, as ions, inside the cell between the anode and cathode. The electrolyte is typically a liquid, such as water or other solvents, with dissolved salts, acids, or alkalis to improve ionic conductivity. Some batteries use solid electrolytes, which are ionic conductors at the cell operating temperature.
Part-2 of this Series will have more knowledge-based details about Lithium-Ion Battery used in Electric Vehicle.
Click to manufacture 7.4kW AC EV Charger.
Click to get Classic Overlay for your Products